Abstract
Introduction Lenalidomide maintenance therapy is associated with a significantly improved progression-free survival in patients with newly diagnosed multiple myeloma; a meta-analysis additionally reported significantly prolonged overall survival. Lenalidomide maintenance is overall well-tolerated; however, fatigue and diarrhea are common side effects. Indeed, the incidence of lenalidomide-associated diarrhea has been documented in up to 25-40% of patients in treated with lenalidomide in clinical trials. The stool microbiome has been linked to sustained responses in patients on lenalidomide maintenance but has not been studied in the setting of lenalidomide-associated diarrhea. Based on a clinical case series with 12 patients (Pawlyn et al, Blood 2014), bile acid malabsorption has been implicated in lenalidomide-associated diarrhea, and, consequently, bile acid binders such as colesevelam have been used in clinical practice. Colesevelam is not taken up systemically and is assumed to not effect lenalidomide pharmacokinetics (PK).
To evaluate the safety and efficacy of a bile acid binder systematically, we conducted a phase 2 investigator-initiated trial of colesevelam for patients with lenalidomide-associated diarrhea. In this trial, stool microbiome studies as well as lenalidomide PK studies of were included to evaluate any potential effect of colesevelam on lenalidomide concentration.
Methods This was a phase 2 single arm open label trial of colesevelam for patients on with lenalidomide-associated diarrhea. Patients with multiple myeloma who were treated with single agent lenalidomide maintenance, and who had grade 1 or more diarrhea per the CTCAE v5 criteria were eligible. Infectious diarrhea was an exclusion criterion and was ruled out in all patients before starting colesevelam. This was a Simon 2-stage pilot study including up to 25 patients. If 5 or more (30%) of the first 16 patients had a clinical response, defined as a decrease in diarrhea by at least 1 grade per CTCAE within the first 4 weeks of treatment, the remaining 9 patients were to be enrolled. Patients were treated with colesevelam daily starting at 1250 mg (2 tablets 625 mg) for a total of 12 weeks. There was a dose adjustment schedule for colesevelam, up to maximum 6 tablets (3750mg) per day and minimum 1 tablet (625 mg) per day, based on grade of diarrhea on initial weekly clinical assessment. Patients were instructed to take colesevelam in the morning and lenalidomide in the evening (at least 4 hours apart). For a subset of 15 patients, pharmacokinetic evaluation of lenalidomide was performed on day 1 and day 8 after starting colesevelam. Stool microbiota was collected at baseline and at the end of trial.
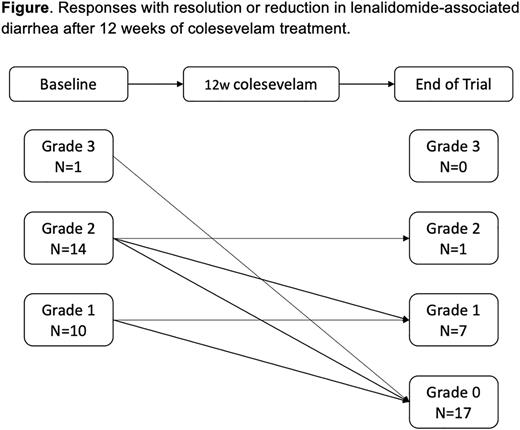
Results The study met the endpoint per the Simon stage 2 criteria, and a total of 25 patients were enrolled. There were 15 women and 10 men with a median age at inclusion of 60 years. At baseline, 1 patient had grade 3 diarrhea, 14 patients had grade 2, and 10 patients had grade 1 diarrhea, respectively.
In total, 22 (88%) of the 25 patients responded with improvement with complete resolution (N=17, 68%) or improvement by 1 grade (N=5, 20%) of diarrhea. Three patients (12%) patients did not respond after treatment with colesevelam, 2 with had ongoing grade 1 and 1 with grade 2 diarrhea (Figure). Five patients (20%) had a dose reduction to colesevelam 625 mg daily and 3 (12%) patients required dose increase to 1875-2500mg (3-4 pills) per day for control of the diarrhea. Side effects from colesevelam included constipation in 2 patients, flatulence in 1 patient, and acid reflux in 1 patient. Pharmacokinetic laboratory studies and stool microbiome analyses are ongoing.
Conclusion In this prospective, single arm clinical trial, colesevelam was safe and highly effective for the control of lenalidomide-associated diarrhea in multiple myeloma patients receiving single agent lenalidomide maintenance. Lenalidomide PK and stool microbiome studies are ongoing and will be reported at the ASH Annual Meeting 2022.
Disclosures
Hultcrantz:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Curio Science LLC: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Intellisphere LLC: Consultancy; Amgen, Daichii Sankyo, Cosette, GSK: Research Funding. Hassoun:Janssen Pharmaceuticals: Research Funding; Takeda: Research Funding; Celgene: Research Funding. Korde:Clinical Care Options, OncLive, Intellisphere: Consultancy; Amgen, Janssen: Research Funding. Mailankody:OncLive: Honoraria; Plexus Communication: Honoraria; Fate Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; Juno Therapeutics: Research Funding; Takeda Oncology: Research Funding; Allogene Therapeutics: Research Funding; Optum Oncology: Consultancy; BioAscend: Consultancy; Janssen Oncology: Consultancy, Research Funding; Evicore: Consultancy; Legend Biotech: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment; Physician Education Resource: Honoraria. Shah:Bristol Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; MJH Lifesciences: Consultancy, Honoraria; ACCC: Honoraria; MashUpMD: Honoraria; Sanofi: Consultancy. Scordo:Omeros Corporation: Consultancy, Research Funding; Angiocrine Bioscience, Inc.: Consultancy, Research Funding; i3Health (CME): Honoraria; McKinsey & Company: Consultancy; Kite - A Gilead Company: Other: Ad-hoc advisory board (past); Amgen, Inc.: Research Funding; Medscape, LCC (CME): Honoraria. Lahoud:MorphoSys, Inc: Membership on an entity's Board of Directors or advisory committees. Landau:Alexion: Other: grants/pending grants; Janssen: Consultancy; Takeda Pharmaceuticals: Consultancy, Other: grants/pending grants; Legend Biotech USA Inc: Consultancy; Caelum Biosciences: Consultancy; Juno: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; Karyopharm: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment; Prothena: Honoraria; Janssen Scientific Affairs, LLC: Other: grants/pending grants. Shah:Janssen: Research Funding; Amgen: Research Funding; Beyond Spring: Research Funding. Giralt:Amgen: Consultancy, Research Funding; Actinuum: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding; Janssen: Consultancy; Jazz Pharmaceutical: Consultancy; Miltenyi: Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy; Omeros: Research Funding; Kite: Consultancy; Spectrum Pharma: Consultancy. Tan:Janssen: Consultancy, Research Funding. Usmani:Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding. Landgren:Amgen: Honoraria, Research Funding; Janssen: Honoraria, Other: Independent Data Monitoring Committee (IDMC) member for clinical trials, Research Funding; Merck & Co., Inc.: Other: Independent Data Monitoring Committee (IDMC) member for clinical trials; Pfizer Inc: Consultancy; Theradex: Other: Independent Data Monitoring Committee (IDMC) member for clinical trials; NCI/NIH: Research Funding; Riney Foundation: Research Funding; Tow Foundation: Research Funding; Rising Tide Foundation: Research Funding; Leukemia & Lymphoma Society: Research Funding; MMRF: Honoraria; Aptitude Health: Honoraria. Lesokhin:BMS: Honoraria; Sanofi: Research Funding; Trillium Therapeutics: Consultancy, Research Funding; Serametrix, inc: Patents & Royalties; Janssen, Pfizer, Iteos, Sanofi, Genmab: Honoraria; Janssen, Pfizer, BMS, Genentech/Roche: Research Funding; Pfizer, Genmab, Sanofi, Iteos, BMS, Janssen: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment; Amgen: Honoraria.
OffLabel Disclosure:
Colesevelam for the treatment of lenalidomide-associated diarrhea
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal